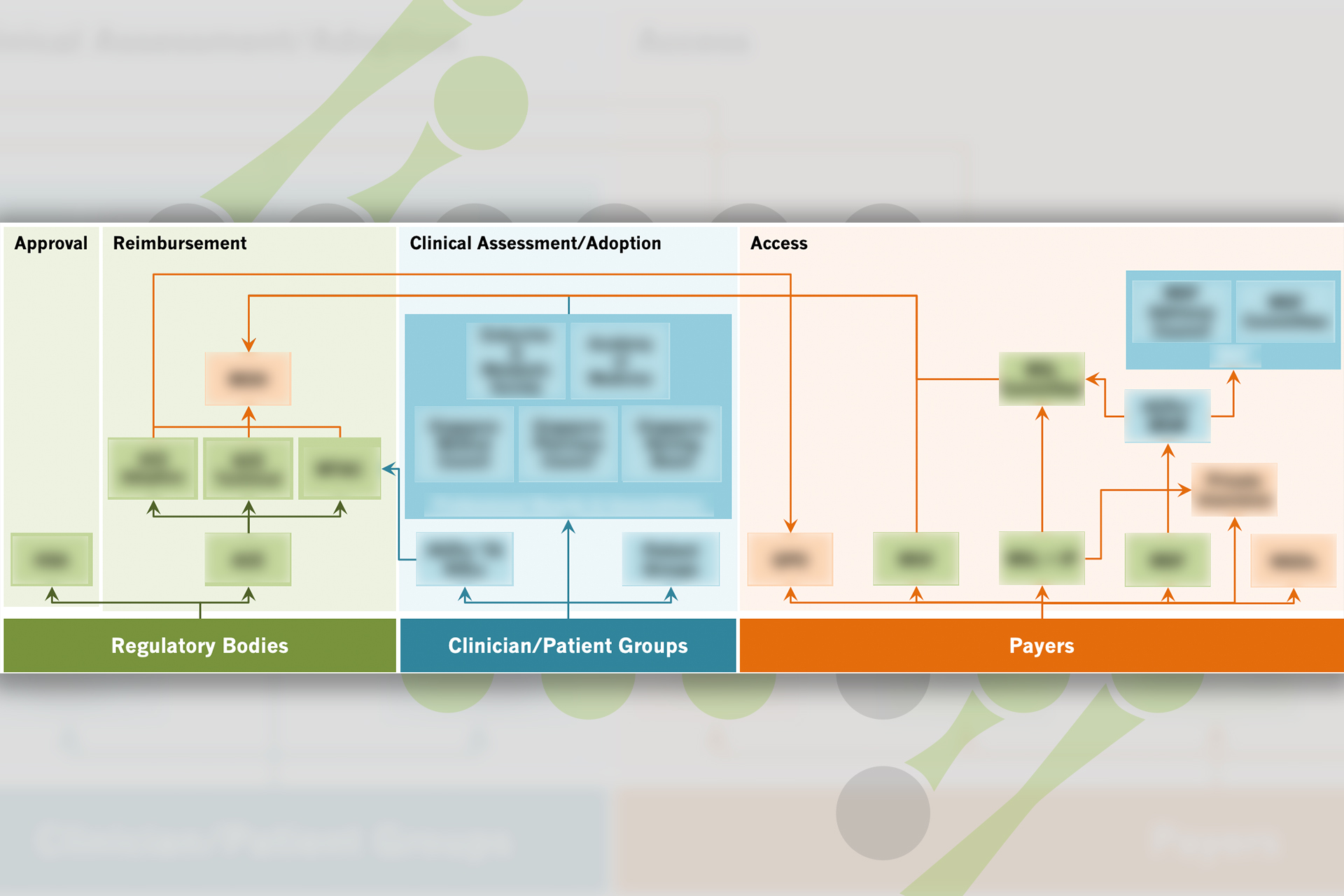
Market landscape analysis to understand the access landscape, funding pathways, reimbursement processes and policies for rare disease across markets and their impacts
Advisory support for access strategy through opportunity, market landscape and analogue assessments
Situation
The client aimed to evaluate the reimbursement and access landscape for rare disease in different APAC markets and gather competitor insights to assess market positioning and pinpoint high-potential markets for future operations.
Geographical Markets

China, Australia, Korea, Taiwan, Singapore, Hong Kong, Thailand, Malaysia, Indonesia, the Philippines
The Approach
Competitive benchmarking analysis by identifying key performance areas and relevant indicator metrics to compare and evaluate competitors’ performance across regions
Value to the Client

Other similar case studies to [Topic]
Case Study 1
[250 characters blurb] Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Case Study 2
[250 characters blurb] Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.
Case Study 3
[250 characters blurb] Lorem ipsum dolor sit amet, consectetur adipiscing elit, sed do eiusmod tempor incididunt ut labore et dolore magna aliqua. Ut enim ad minim veniam, quis nostrud exercitation ullamco laboris nisi ut aliquip ex ea commodo consequat.